 Wacker Neuson powers the Kramer 5055e electric wheel loader with two lead-acid battery-driven motors. Lead-acid batteries, though inexpensive and reliable, are less energy dense than even lithium-ion batteries, which Toyota researchers think they may have recently made headway on replacing with more powerful magnesium batteries.
Wacker Neuson powers the Kramer 5055e electric wheel loader with two lead-acid battery-driven motors. Lead-acid batteries, though inexpensive and reliable, are less energy dense than even lithium-ion batteries, which Toyota researchers think they may have recently made headway on replacing with more powerful magnesium batteries.It’s no secret that the biggest hindrance to today’s battery technology is what the majority of the batteries that power our computers, phones, smartwatches and, increasingly, cars, are made of: lithium.
After 20 years of hybrid car development and selling thousands of Priuses, Toyota may be more familiar with the frustrations of wrangling a highly unstable metal like lithium into a package safe enough to place inside a car than any other company working on advancing battery technology forward.
But things may soon get a bit easier for Toyota, Tesla, Apple and everyone else looking forward to the next step in battery evolution. Thanks to a discovery made by a Toyota scientist whose primary focus, until now, had been hydrogen fuel cell research, we may not be too far away from cheaper, lighter and more energy-dense batteries.
RELATED >> Wacker Neuson is quietly building an entire line of electric loaders, excavators and more
According to a Toyota press release, the company’s researchers have been working quite some time at replacing lithium-ion batteries with a superior magnesium alternative. Toyota says that because lithium can ignite when exposed to air, in order to use the metal to safely power a battery, you have to remove ions from the lithium and place them into graphite rods within the battery.
That means there’s actually no lithium metal in a lithium-ion battery. Not only does this process make lithium-ion batteries expensive to manufacture, it also restricts their energy density. To be sure, lithium-ion batteries are among the more energy dense batteries at our disposal, but its properties greatly restrict our ability to make them much better. It’s why your phone rarely lasts you more than 36 hours and why your electric car can’t quite get 300 miles per charge.
But magnesium, as Rana Mohtadi, principal scientist at Toyota Research North America, explains, is just the opposite. “When magnesium batteries become a reality they will be much smaller than current lithium ion (batteries), they will be cheaper and they will be much safer,” she says.
So why not just make these magnesium batteries if they’re so much better? Well, knowing magnesium is superior to lithium is just one of three pieces to the viable battery puzzle. Batteries require two electrodes— the positive anode and the negative cathode—and an electrolyte, the liquid that allows the flow of ions between the two electrodes.
While magnesium should make a great anode, Toyota researchers first needed to find a compatible electrolyte before they could explore possible cathodes. Enter Mohtadi.
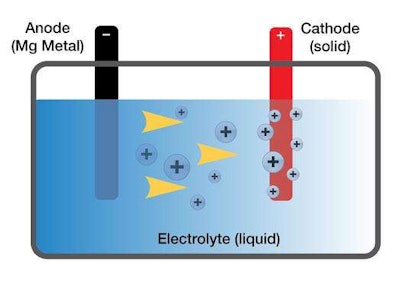
Mohtadi’s theory that these storage materials could work as a magnesium battery electrolyte was correct. Now the automaker’s researchers are hard at work to find a viable cathode.
While a potentially huge breakthrough, Toyota cautions this whole process could take 20 years of research and development before magnesium batteries come to market. However, in the hopes of shortening that R&D period, Toyota wants to make this magnesium electrolyte a standard and is asking other researchers to develop it further.
A cheaper, more energy-dense battery than lithium would not only be embraced by phone, tablet and electric car makers. It would also be a boon to the development of electric heavy equipment such as Wacker Neuson’s zero emissions lineup. WN currently powers its electric wheel loaders, like the Kramer 5055e pictured above, with lead-acid batteries, which has been a proven and reliable power source for fork lifts.
However, lead acid batteries, while inexpensive, are heavier and far less energy dense than even lithium-ion batteries. A magnesium battery would be a definite shot in the arm to the development of such electric equipment.











